In the medical device industry, protecting delicate instrument tips goes beyond just preventing damage. It's about keeping staff and patients safe and maintaining sterility. Whatever delicate instruments you're working with, they need specialized tip protection that can handle transportation, storage, day-to-day handling and sterilization processes.
In this guide, we'll walk through the common uses for medical tip protection, the molding processes used to manufacture these components, and which materials work best for different applications.
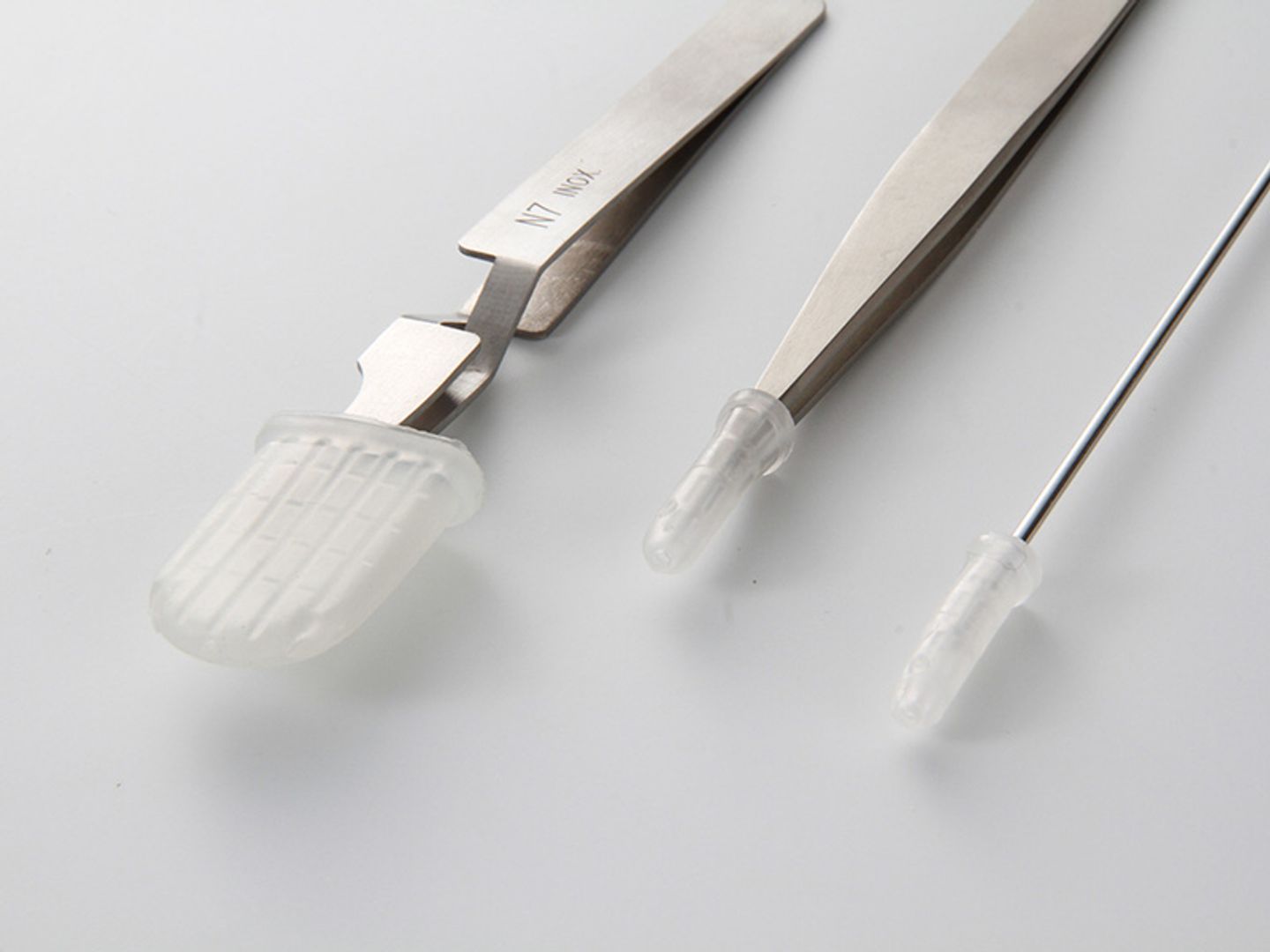
Instrument tip guards serve multiple functions for delicate medical components during transportation, storage and handling. These puncture- and abrasion-resistant caps prevent denting and scratching of delicate tips while also creating a barrier against contaminants and particulates that can compromise instrument performance.
More than just a physical shield, medical tip guards support the sterilization process. Available in both vented and non-vented for full exposure to sterilization, these caps also prevent moisture from being trapped between the tip protector and instrument that can lead to corrosion or bacterial growth.
Instrument caps provide the perfect solution for most medical tips including distal tips, tweezers, forceps and more, protecting the ends of delicate medical instruments while meeting all cleanliness standards.
For medical applications, injection-molded tip protectors offer strength and durability, making them the ideal choice for shipment, storage and handling. The injection molding process allows for the addition of venting directly into the part design, ensuring proper sterilization and moisture evaporation. These tip guards can be manufactured in a variety of sizes to accommodate a full range of instruments and tip dimensions.

Consistency - Even with Complex Parts
Medical device manufacturers require consistency when protecting instruments. Injection molding delivers identical parts, even if complex, that offer features such as vents and channel designs.
High-Volume Production at Scale
Injection molded tools can feature anywhere from a single cavity to hundreds of cavities, enabling the production of many parts during a single manufacturing cycle. With cycle times averaging less than a minute, high volumes of medical tip protection can be achieved.
Volume Requirements
Injection molding is most economical for medium to high-volume production. For smaller quantities of injection molded medical tips, sample kits may provide a more practical solution.
Compression-molded tip protectors offer excellent biocompatibility, making them ideal for sensitive medical applications. Caplugs manufactures these silicone protectors in a Class 8 cleanroom environment, ensuring the highest levels of cleanliness and quality control throughout production.
Medical-Grade Quality
Parts are manufactured in a cleanroom environment using USP Class VI certified medical-grade silicone. This controlled production process ensures cleanliness suitable for direct contact with medical instruments and sterile environments while preventing contamination.
Scalable Production Volumes
Compression molding excels at producing both small prototype runs and large-scale production quantities. Multi-cavity tooling enables high-volume production of multiple parts with consistent quality and minimal material waste.
Cost & Leadtime
Compression molded tooling costs a fraction of injection molded or LIM tooling, with prototype tooling and parts often available within four to five weeks.
Size Customization
Available in nearly 40 catalog sizes and custom options to accommodate a wide range of tip and port dimensions.
Design Limitations
While complex parts are achievable, intricate components present manufacturing challenges and may not be suitable for compression molding.
Vinyl dip molded tip protection offers flexible yet durable protection that helps prevent damage during transport. The vinyl material can also keep medical staff safe from sharp points during handling, addressing both equipment protection and workplace safety concerns.
Certain designs are engineered for instruments with more than one tip, such as forceps, scissors, clamps or dilators, providing protection for complex surgical tools. Venting can also be designed into these parts or added in a secondary operation.
Impact Protection
Vinyl’s thickness provides excellent cushioning against breaking and chipping of medical tips. For applications requiring maximum impact protection, wall thickness can be increased.
Variety of Sizes & Shapes
Vinyl caps and plugs are available in a wide range of fitting types. Select designs are vented to allow for the quick evaporation of moisture or gas during sterilization.
Material Properties & Flexibility
Vinyl material often provides enhanced material characteristics and flexibility for wider applications.
Cost & Leadtime
Vinyl dip molded tooling is a fraction of the cost of Injection molded tooling. Prototype tooling and parts are often available within one to two weeks.
Part Geometry
Due to limitations of vinyl dipped tooling, part geometries do not allow for excessive undercuts or complexity.
Tolerance
Tighter tolerances are often limited to inside dimensions which are controlled by tooling dimensions.
Secondary Operation Challenges
Adding precise features like vent holes can complicate production and drive up costs for secondary operations, but the process works well for most applications.
Thermoplastic polyurethane (TPU) films and sheets can be die-cut into exact shapes for device components. TPU die-cut parts can also be welded to form a tightly sealed pouch.
Design Flexibility
Die-cutting offers complete customization, allowing for a wide range of sizes and shapes based on medical tip design.
No Tooling For Die-Cuts
Die-cuts don't require traditional tooling, helping lower costs and creating faster production turnaround. It is important to note that welding tooling and fixturing are still required when creating sealed pouches.
Material Elasticity Challenges
TPU's elasticity and thickness can present challenges during the cutting process.
When it comes to medical tip protection, material selection makes all the difference. Medical device manufacturers focus on three key priorities: cleanliness, biocompatibility, and sterilization capability when choosing the right material for their needs.
Medical-grade silicone, particularly USP Class VI certified silicone, is an ideal choice for medical instrument tip protectors that require the highest levels of biocompatibility and sterilization. Caplugs manufactures silicone tip protectors using compression molding in a Class 8 cleanroom environment, ensuring cleanliness, consistency and product quality. These tip caps provide the ideal balance of cleanliness, durability and secure fit.
TPE tip protectors can be injection molded for high-volume production of complex tip guards with tight tolerances (both vented and non-vented). The material is durable, lightweight and flexible, with a secure grip for easy installation and removal. Natural color options offer full instrument visibility, enhancing clinician safety. FDA-grade TPE is available, and production can be performed in a Class 8 cleanroom to meet stringent cleanliness requirements.
A sturdy, lightweight material, TPU is often clear, allowing medical staff to visually inspect packaged instruments without having to remove the protective barrier. It can be die-cut into precise shapes and the material can weld easily to create sealed pouches where contamination is a concern. Reinforced edges can be welded around the most critical parts of an instrument.
Vinyl has served the medical device industry for decades as a cost-effective, versatile tip protection material. Vinyl tip guards offer excellent flexibility, durability and ease of installation while providing reliable protection during transport and storage. The material's strength protects staff from sharp points during handling. Caps are available in both vented and non-vented options to meet sterilization needs. Vinyl medical caps are made from FDA-grade material and are suitable for steam and EtO (Ethylene Oxide) sterilization methods.
When catalog solutions don’t meet application requirements, custom and catalog variation medical tip protection is available. Catalog variations can include cutting to specific dimensions, adding slices and holes, pad printing, color changes, as well as the use of different materials, additives or packaging configurations and private labeling packaging as needed.
Whenever possible, existing tooling or tooling bases are utilized to minimize custom tooling and catalog variation costs while still delivering tailored designs.
Whether the requirement involves a minor modification to an existing catalog part or a completely new tip protector design, Caplugs offers the engineering expertise, manufacturing capability and quality control necessary to support complex tip protection applications.
With over 75 years of experience serving more than 1,300 medical customers, including 25 of the top 30 medical device manufacturers, Caplugs understands the critical requirements of medical tip protection. Our solutions are designed to preserve instrument integrity, support sterility and protect against damage and contamination throughout manufacturing, sterilization, shipping and storage.
Caplugs offers a comprehensive range of instrument tip protectors, from ready-to-ship catalog options to fully custom solutions engineered for your specific instruments.
Ready to find the right tip protection for your surgical instruments? Explore our full selection of instrument tip guards or visit our medical solutions page to learn more. For custom designs or technical support, contact our team at sales@caplugs.com or call 1.888.CAPLUGS.
When you need a custom protective part for a unique need, let the largest, most experienced in-house team of design engineers develop it for you from initial design to final production. We offer extensive custom molding solutions to meet your cost, performance, and production requirements